Avidin ISO9001:2015 Certification
Driving Quality Assurance in the Biochemical Industry: A Trusted Leader
Avidin Certification and Quality Assurance: Ensuring Excellence in Avidin Production
In the realm of the life science industry, specifically pharmaceuticals and diagnostics, e-Proteins plays a prominent role in avidin certification. Our central mission revolves around meticulously producing avidin, with a firm commitment to delivering products of the highest quality, surpassing mere compliance with documentation.
ISO-9001:2015 Certification: A Guarantee of Quality
Within e-Proteins, our avidin production system meticulously adheres to ISO-9001:2015 standards, known as a gold standard in quality management. This certification involves independent organizations rigorously auditing our processes. ww.sgs.com grants the ISO9001 certification, which is clear evidence of our unwavering commitment to adhering to the regulations governing both the biotechnological and pharmaceutical sectors. In addition to our unwavering focus on quality, we give top priority to safeguarding and backing up critical data and IT systems that are essential for ensuring the traceability of our production processes.
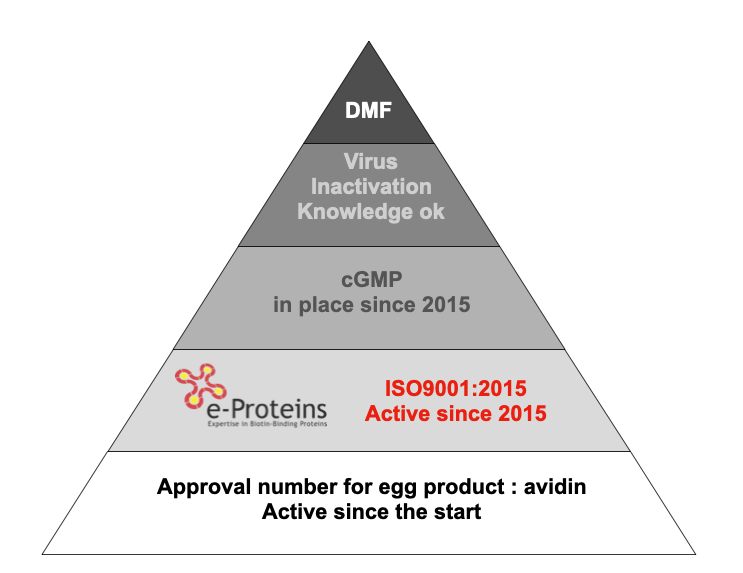
Quality system in place at e-proteins: avidin certification
GMP Avidin Certification: Elevating Quality Standards
For companies currently involved in clinical trials holding an EUDRA number or IND Code, our GMP avidin certification marks a significant turning point. This validation, which receives approval from the Belgian National Health Agency, underlines our dedication to supplying a drug substance that perfectly aligns with its intended usage. The GMP avidin certification process typically extends over approximately nine months, and it incorporates meticulous virus inactivation steps.
In pursuit of futur GMP avidin certification, e-Proteins diligently follows specific guidelines, which include:
- Adhering to the EMEA/CHMP/BWP/398498/2005 guidelines for virus safety evaluation in biotechnological investigational medicinal products.
- Implementing the recommendations outlined in EMA/CHMP/BWP/814397/2011 for the use of porcine trypsin in the manufacture of human biological medicinal products.
- Abiding by the guidelines established in EMA/CHMP/BWP/429241/2013 regarding the use of starting materials and intermediates collected from different sources in the manufacturing of non-recombinant biological medicinal products.
- Implementing the principles laid out in CPMP/BWP/268/95 concerning virus validation studies.
Our watchful online control systems consistently monitor batch records, addressing deviations swiftly. Any discrepancies are meticulously documented in deviation reports, ensuring complete transparency and accountability.
Additional Certifications and Virus Safety Measures
Given the animal origin of avidin, e-Proteins undergoes yearly audits conducted by the National Veteran Agency. These audits serve the crucial purpose of validating our regulatory compliance and securing official approval.
In the arena of virus inactivation and removal, our research has spanned over seven methods. We have thoughtfully selected three of these methods to maintain the maximum level of product activity. This allows us to effectively cater to the unique requirements of our valued customers concerning their drug substances. To attain Virus Inactivation/Removal Certification, a minimum of two methods is deemed essential. This rigorous certification process spans approximately nine months, demanding unwavering dedication and meticulous attention to detail.
Sterile Avidin Certification Process: Ensuring Safety
In our pursuit of sterile certification, e-Proteins forges close collaborations with specialized companies. These companies boast the requisite infrastructure and extensive expertise in the field. This strategic partnership ensures that the certified sterile avidin we produce meets the highest industry standards.
Consequently, our highly skilled e-Proteins pharmacists oversee the release of the certified sterile avidin as a Drug Product. This transformation significantly enhances its suitability for injectable applications during clinical trials. As a result, it plays a pivotal role in bolstering both safety and efficacy, thereby ensuring that our products consistently adhere to the highest standards.
In summary, e-Proteins consistently demonstrates unwavering dedication to upholding the utmost quality standards in avidin production. We further emphasize our commitment through our rigorous certification processes, which strengthen our unwavering pledge to provide exceptional products and services to the life sciences sector.
