Neutralite Avidin
Neutralite avidin : the solution for the non-specific binding issues
Neutralite Avidin, a deglycosylated form of avidin, exhibits a near-neutral isoelectric point of 6.3, significantly reducing non-specific binding and minimizing unwanted interactions in biochemical applications. This unique feature, coupled with its high biotin-binding affinity, makes it ideal for precision assays where specificity is paramount. Moreover, Neutralite Avidin’s reduced molecular weight of 60 kDa contributes to its high solubility, enhancing its efficiency in various biochemical applications. Additionally, it is available in polymerized variants (n-Ultracoat70 for lateral Flow application ), which further enhance its binding capacity. This ability to engage in more effective biotin interaction is particularly beneficial in complex assays, where a higher binding capacity is crucial for obtaining accurate results.
Why Neutralite Avidin Presents a Lower Non-specific Binding
This derivative is relatively free of non-specific binding to cells and DNA due to its lower isoelectric point (pI=6). At this value of pI=6; Lysines remain available. e-proteins can offer custom derivatives with a lower (pI=5,2). The only consequence of this change is the limitations for the derivation and conjugation due to the lack of lysine.
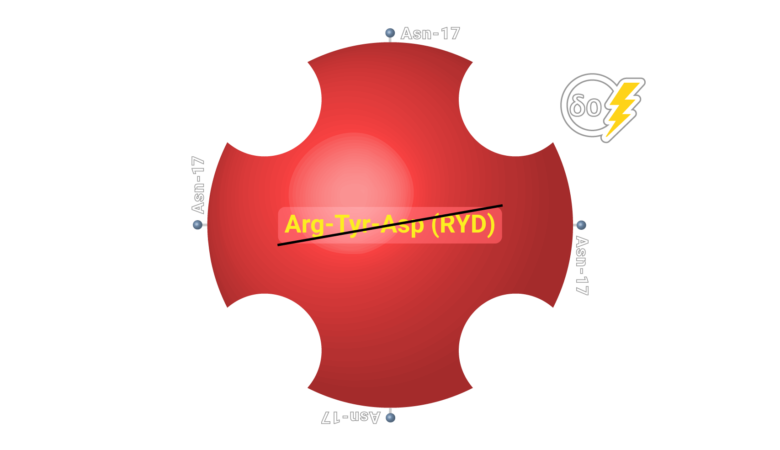
Neutravidin presents high specification in term of non specific binding.
How Neutralite Avidin is a True Replacement for Streptavidin with high Signal/Noise
Neutralite Avidin which has high signal/noise (S/N), is carbohydrate-free and neutral, with the additional benefit of a large number of amino acids available for derivatization and the absence of Arg-Tyr-Asp (RYD) sequences. Altogether, these molecular properties result in the lowest non-specific binding among known biotin-binding proteins such as streptavidin. Its specific activity is close to the theoretical maximum of around 16 µ/mg of protein. Let’s learn more about this novel protein. It is a good replacement for Neutravidin with a comparatively good specific activity.
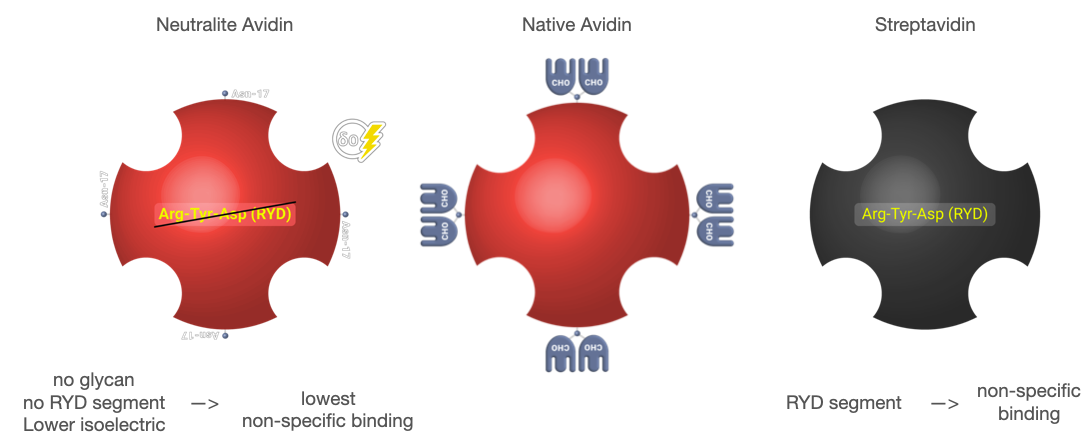
Neutravidin has no glycan, no RYD segment and the lowest pI that offer the lowest Non-Specific Binding
Neutralite Avidin Properties
This innovative protein derivative is a form of lite-avidin with a neutral charge. This neutralization reduces non-specific binding by minimizing the interaction between the protein and charged surfaces or materials. This can be useful in some applications where non-specific binding or high signal/noise is a concern, such as in immunoassays or other biochemical assays where Strepavidin is used.
- This derivative is a deglycosylated and isoelectrically neutral form of Avidin.
- It can replace streptavidin with an advantage because its lower extent of non-specific binding reduces background signals.
- Its structure consists of four identical subunits, each containing a binding site for biotin similar to Avidin.
- Due to its acidic pI, the Neutralize Avidin is relatively free from non-specific binding to cells and DNA.
- Its near-neutral pI (6.3) offers the great advantage of minimizing non-specific adsorption
- As its lysine residues remain available for derivatization or conjugation, Neutralite Avidin can also be conjugated to
- an enzyme (i.e., horseradish peroxidase)
- a fluorescent dye to visualize biotinylated proteins on membranes
This performance of Neutralite Avidin is higher than the Neutravidin, indeed the e-Proteins product shows a higher specific activity and no solubility issue at the pI.
Streptavidin vs Neutralite Avidin
This avidin derivative is a good replacement for Streptavidin with high signal/noise. The product offers solutions to Streptavidin users who are facing issues with their applications:
- No non-specific binding to cell-surface proteins (better than Streptavidin)
- High signal-to-noise ratio in detection systems (better than Streptavidin)
- Highest specificity for biotin binding

Neutralite Avidin Application
This derivative has many applications and some of the most important ones such as:
- Western/Southern/Northern Blotting
- ELISA
- Immunohistochemical staining
- Fluorescence activated cell sorting
Evolution of the Neutralite Avidin Development
In 2012, a new R&D program started to improve product quality to reach higher performance in terms of specific activity, deglycosylated level, isoelectric point, and reproducibility. In 2018, this program successfully ended with NAX (Ultra Neutralite Avidin) with higher performance. Its sales to our direct end-customers started directly.
e-Proteins has launched an R&D project to master the Neutralite Avidin polymerization and better respond to its customers’ expectations of low and high polymerized lots. After 5 years of research, our R&D team has implemented a new approach that avoids the natural olimegorisation of the product. On the other hand, our engineering department has developed a controlled polymerisation process that guarantees the production of Ultracoat70.
For the Neutralite Avidin, e-proteins concentrate their efforts on the deglycolisation level in order to reduce non-specific binding and protein activity as much as possible. As a result, our product has an exceptional specific activity > 15.5 U/mg and successfully competes with steptavidin in many applications with a deglycolisation level of around 95%.
Contact Us if you want to learn more about our product or have any queries. Our company guarantees the best prices for the highest quality.
Prices
Specifications
Details:
| Physicochemistry | NAX100 |
|---|---|
| Purity | 100% |
| Activity (U/mg solid) | > 15,5 |
| Moisture content (% w :w) | < 5.0 % |
| Isoelectric point | 6.2 ± 0.2 |
| Deglycosylation level | ± 90% |
| Dimer | < 10% |
| Solubility (transmittance @650nm 10mg/ml in PBS) | >95% |
| Solubility (transmittance @650nm 15mg/ml in water) | >95% |
| Microbiology | NAX100 |
|---|---|
| Total Bacterial Count in 1 mg | < 1 |
Details:
| Other | NAX100 |
|---|---|
| Appearance | White to off white free flowing lyophilised powder, free of any visible impurities |
| Definition of activity | One Unit activity binds 1 µg of biotin |
| Country of Manufacturing | Belgium |
| Origin | Egg proteins from hens |
Benefit of the neutral isoelectric point
With native avidin, basic proteins stick to negatively charged surfaces by virtue of electrostatic interactions. Although this property can be limited by the addition of salts, most biological tests are conducted under physiological conditions, which are not conducive to eliminating nonspecific absorption. In order to overcome this problem, the isoelectric point of the protein has been reduced to a neutral value under conditions whereby the number of surface lysine residues remains essentially unchanged; the neutral deglycosylated avidin (NeutraLite Avidin) can be used for subsequent derivatization and/or conjugation via lysine residues.
