Nitrolite avidin
Revolutionizing Biotech: The Transformative Power of Nitro- and Iodo-Avidin
Revolutionizing Separation Techniques with Reverse Binding Avidin
Nitro- and iodo-avidin, known for reverse binding avidin, are pivotal in affinity-based separations. Within these compounds, immobilized avidin captures targeted molecules effectively. This ability is crucial for isolating biotinylated molecules and sensitive biological materials. Functioning under optimal pH or with free biotin, these columns remove targets safely and efficiently. Thus, they protect material integrity and allow repeated experimental use. Therefore, nitro- and iodo-avidin, with reverse binding properties, are essential in research demanding reversible biotin binding and intact binding sites.
Unlocking the Potential of Avidin: Reversible Biotin Binding at Its Core
A key feature of nitro- and iodo-avidin is their capacity to maintain high biotin affinity, similar to native avidin, particularly under optimal conditions such as at pH 4. However, their efficiency in binding significantly decreases at higher pH values and becomes almost negligible at pH 10. This behavior is due to the reduction in the pK_a of the phenol group in the modified tyrosine, an essential aspect for forming a hydrogen bond with biotin. Additionally, research indicates that the quaternary structure of these modified proteins is less stable than that of their native counterparts, highlighting a critical balance between structural integrity and functional modification enabled by reverse binding avidin.
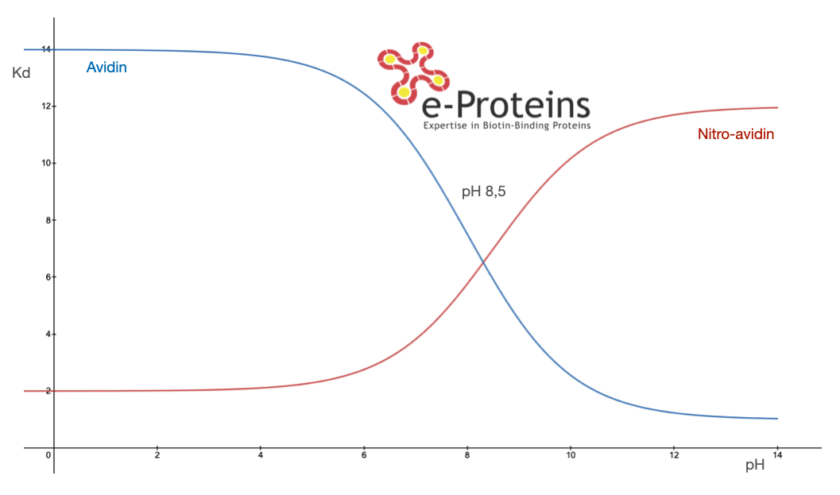
This graph shows the reversible curve of nitro-avidin Vs native Avidin depending on pH
The Science Behind Avidin’s Reversible Binding: A Breakthrough in Biochemistry
The development of nitro- and iodo-avidin involves chemically modifying the tyrosine residue located at the binding site. By altering this residue at the ortho position, the interaction with biotin becomes restricted to lower pH values due to the reduced pK_a of the phenol group. This strategic modification does not eliminate biotin-binding activity but transforms it into a reversible process. Consequently, these proteins have widened their application in avidin-biotin technology, accommodating more sophisticated and sensitive biotechnological processes.
Conclusion
The advancement of nitro- and iodo-avidin signifies a major development in the field of biotin-binding proteins. Their capability for reverse binding avidin within specific pH ranges opens up new opportunities in various biotechnological fields. These include but are not limited to affinity-based separations and the creation of universal avidin columns for repeated use. The innovation brought by nitro- and iodo-avidin underlines the immense potential of chemical modifications in enhancing the versatility and application scope of biotin-binding proteins like avidin and streptavidin, leading to more efficient and targeted biotechnological solutions.
